- 1. Getting Started
- 2. Organic Chemistry 101
-
3. First Semester Topics
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures Guide
- Valence Bond Theory
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Rules of Thumb
- Structural Representations
- Acids/Base and Reactions
- Functional Groups
- Alkanes and Cycloalkanes
- Stereochemistry
-
Alkenes and Addition Reactions
- Application - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation
- Reduction
- Alkynes
- Alcohols and Alkyl Halides
- Aliphatic Substitutions (SN1/SN2)
- Dienes, Allylic and Benzylic systems
-
Structure and Bonding
-
4. Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 5. Spectroscopy - NMR, IR and UV
- 6. Mass Spectrometry
-
7. General Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
-
8. Organic Chemistry Lab
- 9. General Chemistry Lab
- 10. Named Reactions
-
11. Reagents
-
12. Question Of The Day
- 13. Tutorials
- 14. Ask a Question
Clear History
Quick Menu
Functional Groups
Common Functional groups in organic chemistry.
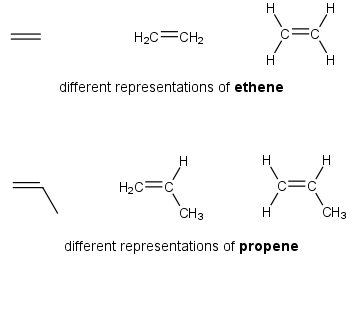
Alkenes (C=C)
The compounds considered to this point have had the general formula CnH2n+2 and are termed the saturated hydrocarbons. The next groups of compounds considered are the alkenes. These compounds have the general formula of CnH2n. It can be seen that they differ from the alkanes in that they have 2 less hydrogen atoms in them. This means that they have a Carbon-Carbon double bond in the molecule. They are named the alkenes and are called unsaturated hydrocarbons.
NOTE: The exception is the cycloalkanes. These compounds will be discussed later.
The simplest alkene is ethene. The next member of the family is propene. The structure of these two compounds is shown below.
 Alkynes (C≡C)
Alkynes (C≡C)
The general formula for the alkynes is CnH2n-2 and these compounds are also considered to be unsaturated. The smallest member of the family is ethyne a.k.a. acetylene. The structure of this compound as well as the next member of the family, propyne, is shown below.
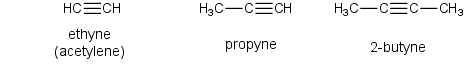
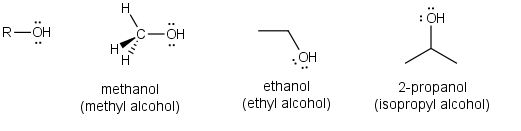
Alcohols (ROH)
Alcohols are the next class of compounds. Most all alcohols are toxic to some extent. In representing the various types of functional groups chemists have adopted the formalism of using R- to represent any saturated alkyl group (those derived from the saturated alkane). This formalism and the smallest member of the family (methanol, CH3OH) are shown below along with the structure of ethanol (CH3CH2OH) and 2-propanol (CH3CHOHCH3). Notice the oxygen has two lone pairs and a hydrogen on it. The chemistry of alcohols is driven by this acidic proton and the lone pairs. They can act like acids or bases.
Amines (RNH2)
The amines are organic compounds that contain nitrogen bonded to a carbon chain(s). The simplest member of the family is methyl amine. This gaseous compound is toxic and highly flammable. Ethyl amine is the next member of the family. Because of the lone pair electrons on the N atom, amines are very basic.
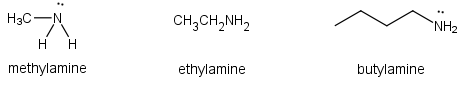
Aldehydes (RCOH)
The smallest aldehyde is methanal (formaldehyde). It is a highly toxic gas. It also is a carcinogen and a teratogen. The next member of the family is ethanal (acetaldehyde).

The C=O double bond in aldehyde's is referred to as a carbonyl group.
Ketones (RCOR)
Ketones also have a carbonyl group, however, in ketones there are carbon containing groups on each side of the carbonyl. The smallest possible ketone is propanone (acetone).

Carboxylic Acids (RCO2H)
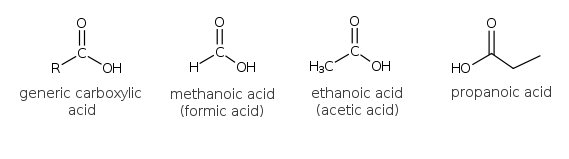
The carboxylic acids also contain a carbonyl group but an -OH (hydroxyl) group is attached to the carbonyl. This is collectively referred to as a carboxyl (or carboxy group), -COOH. The simplest member of this family is methanoic acid (formic acid). Ethanoic acid is the next member of the family. It is also known as acetic acid . A 3% solution of ethanoic acid in water is vinegar.
Esters (RCO2R)
The esters also contain a carbonyl group but an -OR is attached rather than an -OH group as in the carboxylic acids. The simplest member of the family is the methyl derivative of methanoic acid. This is given the name of methyl methanoate or methyl formate. The ester derivatives of ethanoic acid also have a common name, that of acetates. Ethyl acetate (ethyl ethanoate) is a common organic solvent that is found in finger nail polish remover.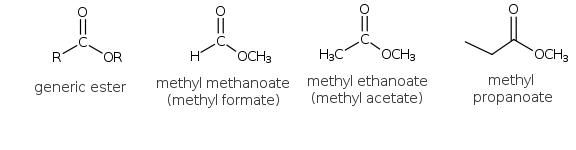
Amides (RCONH2)
Amides have an amine attached to a carbonyl carbon.
Anhydrides - (RCO)2O
Anhydrides are not found in nature and are typically only synthesized in the lab. The most prominent one is ethanoic anhydride (acetic anhydride).

Carboxylic Acid Halides (RCOX)
These compounds are usually considered a safety risk. The low molecular weight members of this family of compounds react vigorously with water to produce highly irritating products (Acids). The compounds have a halide (usually Cl or Br) attached to a carbonyl functional group. Acid halides are not found in nature and are typically only synthesized in the lab.

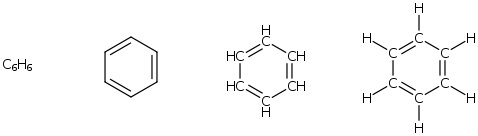
Aromatics
This group encompasses a wide variety of compounds that contain a benzene or benzenoid (i.e. benzene like) component as part of their structure. The structure of benzene is shown below.