- 1. Getting Started
- 2. Organic Chemistry 101
-
3. First Semester Topics
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures Guide
- Valence Bond Theory
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Rules of Thumb
- Structural Representations
- Acids/Base and Reactions
- Functional Groups
- Alkanes and Cycloalkanes
- Stereochemistry
-
Alkenes and Addition Reactions
- Application - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation
- Reduction
- Alkynes
- Alcohols and Alkyl Halides
- Aliphatic Substitutions (SN1/SN2)
- Dienes, Allylic and Benzylic systems
-
Structure and Bonding
-
4. Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 5. Spectroscopy - NMR, IR and UV
- 6. Mass Spectrometry
-
7. General Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
-
8. Organic Chemistry Lab
- 9. General Chemistry Lab
- 10. Named Reactions
-
11. Reagents
-
12. Question Of The Day
- 13. Tutorials
- 14. Ask a Question
Clear History
Quick Menu
MS Fragmentation
Molecular ions fragment and break apart into pieces because they are unstable. Usually its the weakest bonds in molecules that break first. In general the different functional groups fragment in different ways.
Alcohols
Alcohols undergo two common primary fragmentation reactions; α-cleavage and dehydration.
α-cleavage

For example in the following mass spectrum of 3-hexanol an m/z=59 and m/z=73 were observed.

Dehydration
Alcohols will also undergo dehydration expelling water and forming cation radicals. The 3-butanol example above shows a low abundance peak at m/z=84 from loss of water.

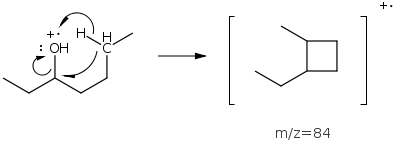
Ethers
Ethers undergo alpha cleavage much like alcohols.
Amines
Nitrogen containing compounds will have a odd mass molecular ion peak if they have an odd number of N atoms and even mass if they have an even number of N atoms.
Amines undergo α-cleavage to form iminium ions (protonated imines).

Ketones, Aldehydes, Esters and Carboxylic Acids
The primary fragmentation of carbonyl containing compounds is α-cleavage and McLafferty rearrangement.
α-cleavage
