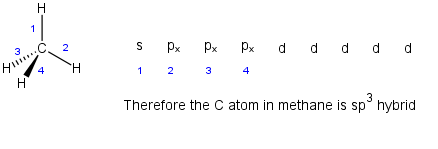- 1. Getting Started
- 2. Organic Chemistry 101
-
3. First Semester Topics
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures Guide
- Valence Bond Theory
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Rules of Thumb
- Structural Representations
- Acids/Base and Reactions
- Functional Groups
- Alkanes and Cycloalkanes
- Stereochemistry
-
Alkenes and Addition Reactions
- Application - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation
- Reduction
- Alkynes
- Alcohols and Alkyl Halides
- Aliphatic Substitutions (SN1/SN2)
- Dienes, Allylic and Benzylic systems
-
Structure and Bonding
-
4. Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 5. Spectroscopy - NMR, IR and UV
- 6. Mass Spectrometry
-
7. General Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
-
8. Organic Chemistry Lab
- 9. General Chemistry Lab
- 10. Named Reactions
-
11. Reagents
-
12. Question Of The Day
- 13. Tutorials
- 14. Ask a Question
Clear History
Quick Menu
Hybridization
What is hybridization, how to determine the hybridization of an atom and why do we bother with it?
What is hybridization?
Organic chemists knew that methane (CH4) had a tetrahedral geometry and that all the C-H bonds were the same length. However, they also knew that methane's structure was inconsistent with the electronic configuration of the C atom.
Recall from general chemistry that we can describe the ground state and valence shell electronic configurations by using Aufbau's principle in which the orbitals (i.e. 1s, 2s, 2px, 2py, 2pz etc.) are filled from the bottom up (i.e. lowest energy first), Pauli exclusion principle (i.e. electrons are paired up in orbitals with opposite spin) and Hund's rule of maximum multiplicity (i.e. degenerate orbitals are given 1 electron until all are half-filled then we pair them up). You should have learned this in high school chemistry and then again in general chemistry as a freshman.
If you do this for C you'd get the valence shell configuration as shown below on left. You'll notice there are only two unpaired electrons (red) in the valence shell configuration.
THERE IS NO WAY FOR IT TO FORM FOUR BONDS!
Something is wrong! We could add energy to the atom and bump one of the 2s electrons into the empty 2p orbital to give an atom with 4 unpaired electrons (excited state below). The energy required would easily be compensated for by the extra bond formed. However, in the excited state one electron is lower in energy than the other three. If this atom were to form a methane molecule one C-H bond would be much shorter than the other three.
AGAIN THIS IS INCONSISTENT WITH THE TRUE STRUCTURE!
However if the 2s and 2p(x,y,z) orbitals could mix together (hybridize) we could potentially make four (4) sp3 hybrid orbitals all of equal energy. This would give a methane structure that is consistent with the true structure of methane. Hybridization occurs because the resulting atom can form 4 bonds which is much more stable than forming only two 2 bonds. Note: The ground state configuration as you learned in gen chem really only applies to bare "naked" atoms with nothing around them. Its the presence of the H atoms that makes the orbitals hybridize.
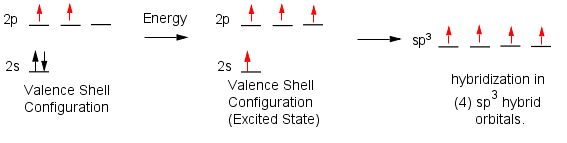
Similarly, we can also have sp2 and sp hybrid atoms.
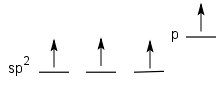
With sp2 hybrid atoms, ones and two p orbitals combine to form three sp2 hybrid orbitals. The one leftover p orbital is available to form a π bond (if it has an electron in it) or it can be unoccupied (i.e. no electrons in it). In the latter case, this forms an important class of molecules known as Lewis acids.
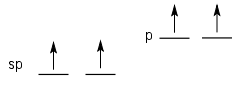
Atoms that are sp hybrid have two leftover p orbitals. These typically can form two π bonds (e.g. as in alkynes).
The different hybridizations and associated geometries are summarized below. As you begin to study organic chemistry you should not only be able to quickly recognize the different hybridizations but also recall the number of non-hybrid orbitals. The non-hybridized orbital will typically become part of a π bond or remain empty.
| Hybridization | Orbitals Involved | # of Hybrid Orbitals | Non hybrdized Orbitals | Electronic Geometry | Angles |
| sp3 | s, px, py, pz | 4 | none | tetrahedral | 109.5 |
| sp2 | s, px, py | 3 | pz | trigonal planar | 120 |
| sp | s, px, | 2 | py, pz | linear | 180 |
How to determine hybridization?
I'm amazed sometimes at how difficult students think determining hybridization is! It's actually quite easy, but I'd bet some poor 2nd-semester students still can't do this.
To determine the hybridization you need to be able to count to at least 5. You also need to know the difference between sigma (σ) and pi (π) bonds. If there is one bond between two atoms its a σ bond. If there are two bonds, one is a σ and one is a π. If there are three bonds, one is a σ and two are π bonds. Now each second-row element can only have (1) s orbitals and (3) p orbitals (i.e. px, py and pz). This is four orbitals, and if each can hold two electrons then the maximum is 8 electrons total (i.e. the octet rule). Atoms in rows beyond the second row can potentially have (1) s, (3) p's, and (5) d's and we have what's called the 18 electron guideline. So you need to count the total number of lone pairs and sigma bonds about the atom of interest and then simply count up through the available orbitals. Let's try some simple examples.
Example 1