- 1. Getting Started
-
2.
First Semester Topics
-
General Chemistry Review
- Introduction
- Electron Configurations of Atoms
- QM Description of Orbitals
- Practice Time - Electron Configurations
- Hybridization
- Strategy to Determine Hybridization
- Practice Time! - Hybridization
- Formal Charge
- Practice Time - Formal Charge
- Acids-bases
- Practice Time - Acids and Bases
- Hydrogen Bonding is a Verb!
- Progress Pulse
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Electron Configurations Tutorial
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures
- Valence Bond Theory
- Valence Bond Theory Tutorial
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Structural Representations
- Progress Pulse
-
Acids/Base and Reactions
- Reactions
- Reaction Arrows: What do they mean?
- Thermodynamics of Reactions
- Acids Intro
- Practice Time! Generating a conjugate base.
- Lewis Acids and Bases
- pKa Scale
- Practice Time! pKa's
- Predicting Acid-Base Reactions from pKa
- Structure and Acidity
- Structure and Acidity II
- Practice Time! Structure and Acidity
- Curved Arrows and Reactions
- Nucleophiles
- Electrophiles
- Practice Time! Identifying Nucleophiles and Electrophiles
- Mechanisms and Arrow Pushing
- Practice Time! Mechanisms and Reactions
- Energy Diagrams and Reactions
- Practice Time! - Energy Diagrams
- Progress Pulse
- Introduction to Retrosynthesis
-
Alkanes and Cycloalkanes
- Introduction to Hydrocarbons and Alkanes
- Occurrence
- Functional Groups
- Practice Time! Functional Groups.
- Structure of Alkanes - Structure of Methane
- Structure of Alkanes - Structure of Ethane
- Naming Alkanes
- Practice Time! Naming Alkanes
- Relative Stability of Acyclic Alkanes
- Physical Properties of Alkanes
- Ranking Boiling Point and Solubility of Compounds
- Conformations of Acyclic Alkanes
- Practice Time! Conformations of acyclic alkanes.
- Conformations of Cyclic Alkanes
- Naming Bicyclic Compounds
- Stability of Cycloalkane (Combustion Analysis)
- Degree of Unsaturation
-
Stereochemistry
- Enalapril in ACE
- Constitutional and Stereoisomers
- Chirality or Handedness
- Drawing a Molecules Mirror Image
- Exploring Mirror Image Structures
- Enantiomers
- Drawing Enantiomers
- Practice Time! Drawing Enantiomers
- Identifying Chiral Centers
- Practice Time! Identifying Chiral Molecules
- CIP (Cahn-Ingold-Prelog) Priorities
- Determining R/S Configuration
- Diastereomers
- Meso Compounds
- Fischer Projections
- Fischer Projections: Carbohydrates
- Measuring Chiral Purity
- Practice Time! - Determining Chiral Purity and ee
- Chirality and Drugs
- Chiral Synthesis
- Prochirality
- Converting Fischer Projections to Zig-zag Structures
- Practice Time! - Assigning R/S Configurations
-
Alkenes and Addition Reactions
- The Structure of Alkenes
- Alkene Structure - Ethene
- Naming Alkenes
- Health Insight - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation and Reduction in Organic Chemistry
- Calculating Oxidation States of Carbon
- Identifying oxidation and reduction reactions
- Practice Time - Oxidation and Reduction in Organic
- Oxidation
- Reduction
- Capsaicin
- Alkynes
- Alcohols and Alkyl Halides
- Substitutions (SN1/SN2) and Eliminations (E1/E2)
- Dienes, Allylic and Benzylic systems
-
General Chemistry Review
-
3.
Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers and Cryptands
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 4. Spectroscopy - NMR, IR and UV
-
5.
Special Topics
-
Acyl Transfer Reagents and Catalysts
-
Asymmetric Synthesis
- General Principles
- Substitutions
-
1,2 and 1,4 Additions
- Aldol and Micheal Additions
- Chiral Pool Synthesis
- Reductions and Hydroborations
- Crystallization-Induced Asymmetric Transformation (CIAT)
- Oxidations
- Cycloadditions
- Dynamic Kinetic Resolution (DKR)
- Curtin-Hammett Principle
- Organocatalysis
- Double Stereodifferentiation
- Felkin Ahn JSMol
- Felkin Ahn JSMol 2
- Felkin-Anh Model
- Kinetic Resolution
- The Burgi-Dunitz Trajectory
- Zimmerman-Traxler Model
- Zimmerman-Traxler TS1
- Complex Induced Proximity Effect (CIPE)
- Introduction to Drug Development
- Kinetic Isotope Effects
- Organometallic Chemistry
- Pericyclic Reactions
-
Acyl Transfer Reagents and Catalysts
-
6.
General Chemistry
- General Chemistry Lab
- Calculator Tips for Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Practice time! Empirical and Molecular Formulae
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
- Practice Time! Le Chatelier's Principle
- 7. Organic Chemistry Lab
-
8.
Question Of The Day
- 9. Tools and Reference
-
10.
Tutorials
- Using OpenOChem's 2D Editor
- Reaction Mechanisms (introduction)
- Factor Labels
- Acetylides and Synthesis
- Drawing Cyclohexane Chair Structures
- Drawing Lewis Structures
- Aromaticity Tutorial
- Common Named Aromatics (Crossword Puzzle)
- Functional Groups (Flashcards)
- Alkyl and Alkenyl Groups
- Valence Bond Theory
Clear History
Chemical Shift
Shielding
If you were to take just a bare proton by itself (no electrons around it) and placed it in a magnetic field (Bo), the proton would experience or "feel" the entire field strength Bo. However, most nuclei (H's and C's) are embedded in orbitals with electrons around them. Nuclei with more electrons around them feel less of the field Bo. A simplified picture that is useful for remembering this effect is "shielding". The electron density behaves like a shield, shielding the nuclei from the magnetic field. More electron density means more shielded and less electron density means deshielded. While this is a nice analogy that works well, the "real" physics is a bit more advanced.
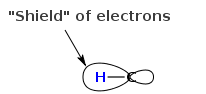
Luckily for organic chemists different types of protons have different electron density, and we can tell them apart when we take an HNMR.
For example in chloroethane, there are 2 nonequivalent types of protons, colored blue and red.
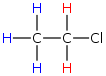
Which protons would you think to have greater electron density around them, red or blue? If you recall, chlorine withdraws electron density by induction and induction depends upon the distance. The red protons feel the effect of the withdrawing group since they are closer and therefore have lower electron density. We would say the red protons are deshielded. The blue protons are further away and therefore have more electron density around them and are more shielded.
Chemical Shift is a measure of shielding!
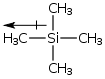
Shielding is expressed in terms of a quantity called chemical shift (δ) and has units of parts per million (ppm) of the field strength. Chemical shift value are relative to an internal standard which is normally tetramethylsilane (TMS). The protons on TMS are more shielded than most other protons encountered in organic chemistry. Silicone is less electronegative than carbon, and also has electrons in diffuse d orbitals. TMS is given a chemical shift of 0 ppm and all other protons are relative to this. A useful mnemonic is TMS stands for "The Most Shielded". The most shielded end of the spectra is on the right where TMS is at 0 ppm, while the deshielded end is to the left on an NMR spectra.
|
|
|
tetramethylsilane (TMS) |
Proton chemical shifts of protons can vary from about -1 (highly shielded) to 13 (highly deshielded).
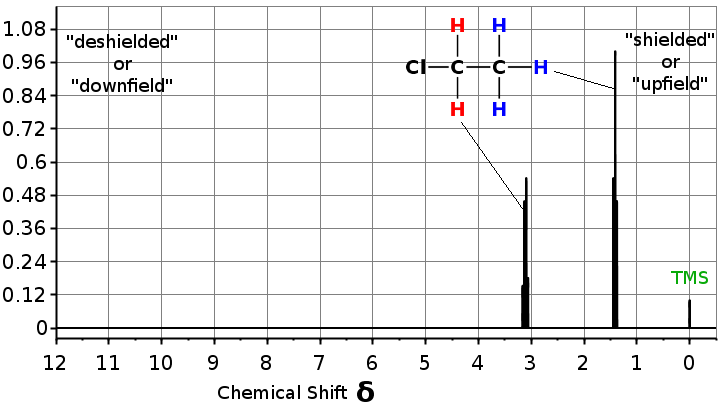
The chemical shifts for many types of protons have been tabulated. There are few very common proton chemical shifts you should commit to memory and they are listed below.
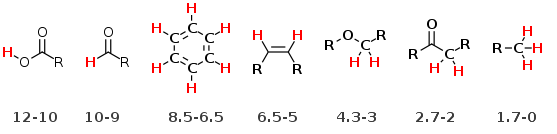
The following more inclusive table or a the table from you textbook book should also prove useful.
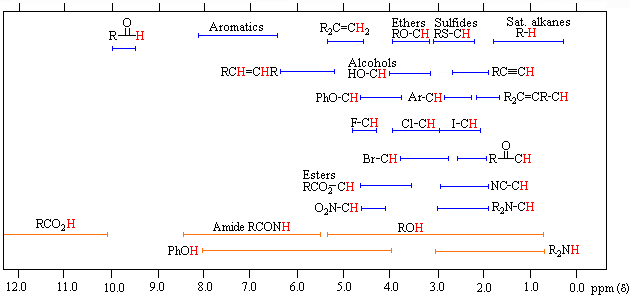
Factors Affecting Chemical Shift
There are a number of factors which influence chemical shift.
- Inductive effects from adjacent EWG
- Magnetic anisotropy in structures with π systems
- Hydrogen bonding
Inductive effects from adjacent EWG.
The chemical shift of a proton is influenced by the electronegativity of the atoms attached to the carbons it is attached to. The protons of fluoromethane have a chemical shift of 4.3 ppm, due to the decreased electron density around its protons from the highly electronegative fluorine.
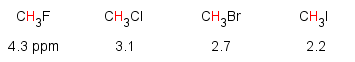
This effect is additive...the more electronegative groups, the more deshielding and the increase in chemical shift.
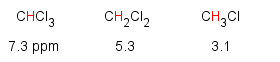
A similar trend is observed going across the periodic table.
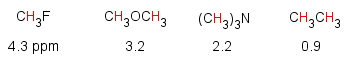
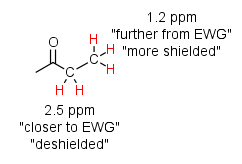
This effect depends on the distance from the EWG. For example, the protons adjacent to the carbonyl group are deshielded more so than the protons two carbons from it.
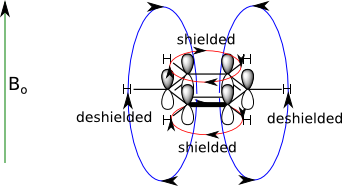
Magnetic Anisotropy
Electrons in molecules with π systems (e.g. aromatics, alkenes, alkynes and aldehydes) can circulate creating a ring current(red lines below) when place in the applied magnet field Bo. The ring current then induces or creates an induced magnetic field (blue lines). As a result, the protons around the outside of the ring are deshielded since the field lines oppose the Bo field in that region. Thus aromatic protons have a chemical shift of about 6.5-8.5 ppm. A similar effect explains the highly deshielded protons observed in aldehydes (9-10 ppm), carboxylic acid (-OH 10-12ppm), and alkenes (5-6.5 ppm)
Hydrogen Bonding
Hydrogen bonding causes the deshielding of protons. Since hydrogen bonding effects are concentration and temperature-dependent you can usually recognize which protons are involved in hydrogen bonding by changing concentration or temperature. With the exception of carboxylic acid OH groups, protons attached to oxygen and nitrogen generally resonate between 0.5 - 5 ppm.