- 1. Getting Started
- 2. Organic Chemistry 101
-
3. First Semester Topics
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures Guide
- Valence Bond Theory
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Rules of Thumb
- Structural Representations
- Acids/Base and Reactions
- Functional Groups
- Alkanes and Cycloalkanes
- Stereochemistry
-
Alkenes and Addition Reactions
- Application - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation
- Reduction
- Alkynes
- Alcohols and Alkyl Halides
- Aliphatic Substitutions (SN1/SN2)
- Dienes, Allylic and Benzylic systems
-
Structure and Bonding
-
4. Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 5. Spectroscopy - NMR, IR and UV
- 6. Mass Spectrometry
-
7. General Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
-
8. Organic Chemistry Lab
- 9. General Chemistry Lab
- 10. Named Reactions
-
11. Reagents
-
12. Question Of The Day
- 13. Tutorials
- 14. Ask a Question
Clear History
Quick Menu
Formal Charge
Formal charge is a way for us to keep track of electrons. In organic chemistry, you will not only need to calculate formal charges but also understand what they mean and how to use them. You will use formal charges to understand structures, intermediates, and reaction mechanisms.
Calculating formal charge
Let us go over the textbook definition first! The formal charge is usually defined as follows;
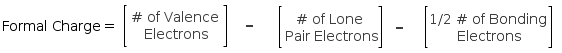
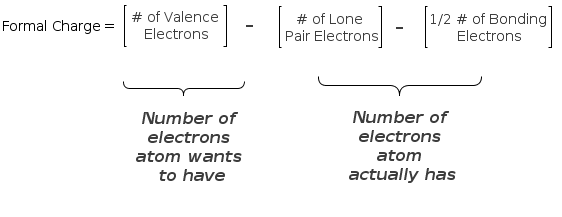
You simply subtract the number of lone pair electrons and 1/2 the bonding electrons (or simply the number of bonds) from the number of valence electrons. Let's try it for ourselves.
What does it really mean?
First of all, there is no reason to memorize the above equation if you realize that formal charge is simply the number of electrons an atom wants to have minus the number of electrons it actually has (or owns). You'll notice that we only subtract half the bonding electrons. Since these are covalent bonds the electrons are shared and the carbon only owns one of the two electrons in each bond, while the H atom owns the other electron.
Limitations of Formal Charge
Calculate the formal charge on the nitrogen atom in the following structure.
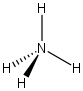
From the periodic table you see that nitrogen has 5 valence electrons (i.e. it wants to have 5 electrons around it). The nitrogen in the structure above has only 4 electrons about it that it owns, so the formal charge is 5 - 4 = +1 and we would write the structure as follows. This is called an ammonium ion.
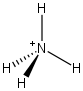
Let us compare the methyl carbocation and ammonium ion to gain an appreciation of what's going on.

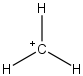
Think about why each structure has a positive formal charge. In the case of the ammonium ion, it is because the nitrogen atom is sharing its lone pair electrons with hydrogen (proton). On the other hand, the methyl cation just does not have enough electrons. Because of this, they behave differently. For example, something that likes a positive charge (i.e. a nucleophile) will attack the carbon atom of the methyl cation. However, a nucleophile will never attack the positive nitrogen atom.
Common patterns
While it is important to be able to calculate the formal charge under any circumstances, it is also helpful to notice when atoms, which are common to organic chemistry (such as carbon, nitrogen, and oxygen), would be expected to have a formal charge. For instance, you may have noticed that neutral carbon always makes four bonds. Similarly neutral nitrogen makes three bonds and neutral oxygen makes two bonds. If these atoms make a different number of bonds other than the numbers just listed, they will have a formal charge. If you train your brain to easily recognize these patterns, then writing structures for intermediates and reaction mechanisms will be easier.
Try completing the following table which summarizes some of these patterns: