- 1. Getting Started
-
2.
First Semester Topics
-
General Chemistry Review
- Introduction
- Electron Configurations of Atoms
- QM Description of Orbitals
- Practice Time - Electron Configurations
- Hybridization
- Strategy to Determine Hybridization
- Practice Time! - Hybridization
- Formal Charge
- Practice Time - Formal Charge
- Acids-bases
- Practice Time - Acids and Bases
- Hydrogen Bonding is a Verb!
- Progress Pulse
-
Structure and Bonding
- Chemical Intuition
- Difficulty-in-organic-chemistry
- Atomic Orbitals
- Electron Configurations of Atoms
- Electron Configurations Tutorial
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures
- Valence Bond Theory
- Valence Bond Theory Tutorial
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Structural Representations
- Progress Pulse
-
Acids/Base and Reactions
- Reactions
- Reaction Arrows: What do they mean?
- Thermodynamics of Reactions
- Acids Intro
- Practice Time! Generating a conjugate base.
- Lewis Acids and Bases
- pKa Scale
- Practice Time! pKa's
- Predicting Acid-Base Reactions from pKa
- Structure and Acidity
- Structure and Acidity II
- Practice Time! Structure and Acidity
- Curved Arrows and Reactions
- Nucleophiles
- Electrophiles
- Practice Time! Identifying Nucleophiles and Electrophiles
- Mechanisms and Arrow Pushing
- Practice Time! Mechanisms and Reactions
- Energy Diagrams and Reactions
- Practice Time! - Energy Diagrams
- Progress Pulse
- Introduction to Retrosynthesis
-
Alkanes and Cycloalkanes
- Introduction to Hydrocarbons and Alkanes
- Occurrence
- Functional Groups
- Practice Time! Functional Groups.
- Structure of Alkanes - Structure of Methane
- Structure of Alkanes - Structure of Ethane
- Naming Alkanes
- Practice Time! Naming Alkanes
- Relative Stability of Acyclic Alkanes
- Physical Properties of Alkanes
- Ranking Boiling Point and Solubility of Compounds
- Conformations of Acyclic Alkanes
- Practice Time! Conformations of acyclic alkanes.
- Conformations of Cyclic Alkanes
- Naming Bicyclic Compounds
- Stability of Cycloalkane (Combustion Analysis)
- Degree of Unsaturation
-
Stereochemistry
- Enalapril in ACE
- Constitutional and Stereoisomers
- Chirality or Handedness
- Drawing a Molecules Mirror Image
- Exploring Mirror Image Structures
- Enantiomers
- Drawing Enantiomers
- Practice Time! Drawing Enantiomers
- Identifying Chiral Centers
- Practice Time! Identifying Chiral Molecules
- CIP (Cahn-Ingold-Prelog) Priorities
- Determining R/S Configuration
- Diastereomers
- Meso Compounds
- Fischer Projections
- Fischer Projections: Carbohydrates
- Measuring Chiral Purity
- Practice Time! - Determining Chiral Purity and ee
- Chirality and Drugs
- Chiral Synthesis
- Prochirality
- Converting Fischer Projections to Zig-zag Structures
- Practice Time! - Assigning R/S Configurations
-
Alkenes and Addition Reactions
- The Structure of Alkenes
- Alkene Structure - Ethene
- Naming Alkenes
- Health Insight - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation and Reduction in Organic Chemistry
- Calculating Oxidation States of Carbon
- Identifying oxidation and reduction reactions
- Practice Time - Oxidation and Reduction in Organic
- Oxidation
- Reduction
- Capsaicin
- Alkynes
- Alcohols and Alkyl Halides
- Substitutions (SN1/SN2) and Eliminations (E1/E2)
- Dienes, Allylic and Benzylic systems
-
General Chemistry Review
-
3.
Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Effect on Rate and Orientation
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Steric Considerations
- Synthesizing Poly-substituted Benzene's
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers and Cryptands
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
- 4. Spectroscopy - NMR, IR and UV
-
5.
Special Topics
-
Acyl Transfer Reagents and Catalysts
-
Asymmetric Synthesis
- General Principles
- Substitutions
-
1,2 and 1,4 Additions
- Aldol and Micheal Additions
- Chiral Pool Synthesis
- Reductions and Hydroborations
- Crystallization-Induced Asymmetric Transformation (CIAT)
- Oxidations
- Cycloadditions
- Dynamic Kinetic Resolution (DKR)
- Curtin-Hammett Principle
- Organocatalysis
- Double Stereodifferentiation
- Felkin Ahn JSMol
- Felkin Ahn JSMol 2
- Felkin-Anh Model
- Kinetic Resolution
- The Burgi-Dunitz Trajectory
- Zimmerman-Traxler Model
- Zimmerman-Traxler TS1
- Complex Induced Proximity Effect (CIPE)
- Introduction to Drug Development
- Kinetic Isotope Effects
- Organometallic Chemistry
- Pericyclic Reactions
-
Acyl Transfer Reagents and Catalysts
-
6.
General Chemistry
- General Chemistry Lab
- Calculator Tips for Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Practice time! Empirical and Molecular Formulae
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
- Practice Time! Le Chatelier's Principle
- 7. Organic Chemistry Lab
-
8.
Question Of The Day
- 9. Tools and Reference
-
10.
Tutorials
- Using OpenOChem's 2D Editor
- Reaction Mechanisms (introduction)
- Factor Labels
- Acetylides and Synthesis
- Drawing Cyclohexane Chair Structures
- Drawing Lewis Structures
- Aromaticity Tutorial
- Common Named Aromatics (Crossword Puzzle)
- Functional Groups (Flashcards)
- Alkyl and Alkenyl Groups
- Valence Bond Theory
Clear History
Zimmerman-Traxler Model
The Zimmerman-Traxler Transition State: Understanding Stereochemical Outcomes
The Zimmerman-Traxler transition state is a cornerstone concept in organic chemistry that helps explain the stereochemical outcomes of certain reactions, particularly aldol reactions. This model proposes a six-membered chair-like cyclic structure that minimizes steric hindrance and optimizes orbital alignment in the transition state, thereby influencing the relative and absolute stereochemistry of the reaction products.
The Role of the Zimmerman-Traxler Model in Aldol Reactions
The aldol reaction involves the addition of an enolate (formed from a ketone or aldehyde) to the carbonyl group of another molecule. This reaction forms a β-hydroxy carbonyl compound, often with one or more stereocenters. The Zimmerman-Traxler model provides a framework to predict how substituents on the reactants control the stereochemistry of these new stereocenters.
Key Features of the Transition State
-
Cyclic Chair-Like Geometry:
- The transition state adopts a six-membered chair conformation, which resembles the chair conformation of cyclohexane.
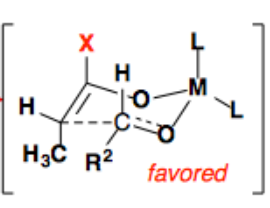
- This geometry minimizes strain and allows for the overlap of the enolate's π-orbital with the electrophile's carbonyl π* orbital.
- The transition state adopts a six-membered chair conformation, which resembles the chair conformation of cyclohexane.
-
Facial Selectivity:
- The enolate and carbonyl group can approach each other in different orientations, depending on steric and electronic factors.
- The larger substituents on both the enolate and the electrophile typically occupy the equatorial positions in the chair-like transition state, reducing steric clashes.
-
Syn vs. Anti Products:
- The orientation of substituents in the transition state dictates whether the reaction forms a syn or anti product.
- For example, in reactions involving Z-enolates, the major product is typically the syn diastereomer, as this minimizes steric interactions in the transition state.
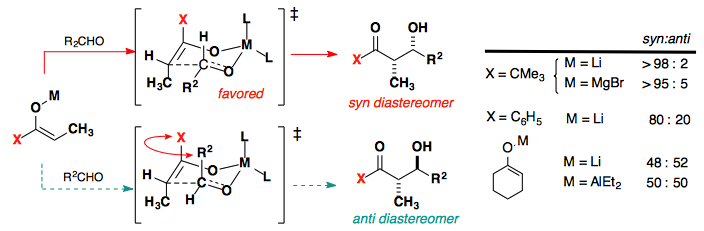
Detailed Example: Aldol Reaction Between Acetone and Benzaldehyde
Consider the base-catalyzed aldol reaction where acetone serves as the enolate donor and benzaldehyde as the electrophile. The reaction proceeds as follows:
-
Formation of the Enolate: A base deprotonates acetone to generate an enolate ion, which acts as a nucleophile.
-
Nucleophilic Attack in the Transition State:
- The enolate aligns with benzaldehyde in a six-membered chair-like transition state.
- The phenyl group of benzaldehyde typically prefers an equatorial position to minimize steric interactions.
- The stereochemistry of the product depends on the alignment of the substituents in this transition state.
-
Formation of the Product: The reaction yields a β-hydroxy ketone. The stereochemical outcome (syn or anti) depends on whether the enolate is in the Z- or E-configuration, which affects the substituent positions in the chair.
General Applications Beyond Aldol Reactions
The Zimmerman-Traxler transition state isn't limited to aldol reactions. It also applies to other reactions involving six-membered cyclic transition states, such as:
- Michael Additions: Involving enolates attacking electron-deficient alkenes.
- Crotylation Reactions: Where allylic groups add to carbonyl compounds with stereochemical control.
Why Is This Model Important?
-
Predicting Diastereoselectivity: The model helps chemists predict whether a reaction will favor a syn or anti product, especially in aldol and related reactions.
-
Designing Stereoselective Reactions: By understanding the transition state, chemists can design reactions with better control over stereochemical outcomes. For example, the use of specific bases or catalysts can favor Z- or E-enolates, altering the product distribution.
-
Rationalizing Observed Outcomes: In complex organic synthesis, the Zimmerman-Traxler model provides a framework to rationalize why certain stereoisomers dominate in a reaction.
Visualization of the Transition State
A diagram of the Zimmerman-Traxler transition state can illustrate how the reactants align during the reaction. Below is a simplified description:
- The enolate forms the "base" of the chair, with its negatively charged oxygen atom coordinating with a Lewis acid (e.g., a metal cation or hydrogen bonding).
- The electrophilic carbonyl group occupies a position where its substituents minimize steric hindrance.
- The substituents on the chair adopt axial or equatorial positions based on their sizes.
|
SYN |
ANTI
|
Practice Problem: Predicting the Major Product
Question:
Predict the major product of the aldol reaction between propanal (as the electrophile) and the Z-enolate of 2-butanone. Provide the expected stereochemistry of the β-hydroxy ketone product.
Solution Outline:
- Draw the enolate and propanal, and construct a six-membered chair-like transition state.
- Orient the substituents to minimize steric hindrance (e.g., the methyl group of 2-butanone enolate in an equatorial position).
- Predict the product stereochemistry (e.g., syn product for the Z-enolate).