- 1. Getting Started
-
2.
First Semester Topics
-
General Chemistry Review
- Introduction
- Electron Configurations of Atoms
- QM Description of Orbitals
- Practice Time - Electron Configurations
- Hybridization
- Closer Look at Hybridization
- Strategy to Determine Hybridization
- Practice Time! - Hybridization
- Formal Charge
- Practice Time - Formal Charge
- Acids-bases
- Practice Time - Acids and Bases
- Hydrogen Bonding is a Verb!
- Progress Pulse
-
Structure and Bonding
- Chemical Intuition
- From Quantum Mechanics to the Blackboard: The Power of Approximations
- Atomic Orbitals
- Electron Configurations of Atoms
- Electron Configurations Tutorial
- Practice Time - Structure and Bonding 1
- Lewis Structures
- Drawing Lewis Structures
- Valence Bond Theory
- Valence Bond Theory Tutorial
- Hybridization
- Polar Covalent Bonds
- Formal Charge
- Practice Time - Structure and Bonding 2
- Curved Arrow Notation
- Resonance
- Electrons behave like waves
- MO Theory Intro
- Structural Representations
- Progress Pulse
-
Acids/Base and Reactions
- Reactions
- Reaction Arrows: What do they mean?
- Thermodynamics of Reactions
- Acids Intro
- Practice Time! Generating a conjugate base.
- Lewis Acids and Bases
- pKa Scale
- Practice Time! pKa's
- Predicting Acid-Base Reactions from pKa
- Structure and Acidity
- Structure and Acidity II
- Practice Time! Structure and Acidity
- Curved Arrows and Reactions
- Nucleophiles
- Electrophiles
- Practice Time! Identifying Nucleophiles and Electrophiles
- Mechanisms and Arrow Pushing
- Practice Time! Mechanisms and Reactions
- Energy Diagrams and Reactions
- Practice Time! - Energy Diagrams
- Progress Pulse
- Introduction to Retrosynthesis
-
Alkanes and Cycloalkanes
- Introduction to Hydrocarbons and Alkanes
- Occurrence
- Functional Groups
- Practice Time! Functional Groups.
- Structure of Alkanes - Structure of Methane
- Structure of Alkanes - Structure of Ethane
- Naming Alkanes
- Practice Time! Naming Alkanes
- Alkane Isomers
- Relative Stability of Acyclic Alkanes
- Physical Properties of Alkanes
- Ranking Boiling Point and Solubility of Compounds
- Conformations of Acyclic Alkanes
- Practice Time! Conformations of acyclic alkanes.
- Conformations of Cyclic Alkanes
- Naming Bicyclic Compounds
- Stability of Cycloalkane (Combustion Analysis)
- Degree of Unsaturation
-
Stereochemistry
- Enalapril in ACE
- Constitutional and Stereoisomers
- Chirality or Handedness
- Drawing a Molecules Mirror Image
- Exploring Mirror Image Structures
- Enantiomers
- Drawing Enantiomers
- Practice Time! Drawing Enantiomers
- Identifying Chiral Centers
- Practice Time! Identifying Chiral Molecules
- CIP (Cahn-Ingold-Prelog) Priorities
- Determining R/S Configuration
- Diastereomers
- Meso Compounds
- Fischer Projections
- Fischer Projections: Carbohydrates
- Measuring Chiral Purity
- Practice Time! - Determining Chiral Purity and ee
- Chirality and Drugs
- Chiral Synthesis
- Prochirality
- Converting Fischer Projections to Zig-zag Structures
- Practice Time! - Assigning R/S Configurations
-
Alkenes and Addition Reactions
- The Structure of Alkenes
- Alkene Structure - Ethene
- Physical Properties of Alkenes
- Naming Alkenes
- Health Insight - BVO (Brominated Vegetable Oil)
- E/Z and CIP
- Stability of Alkenes
- H-X Addition to Alkenes: Hydrohalogenation
- Practice Time - Hydrohalogenation
- X2 Addition to Alkenes: Halogenation
- HOX addition: Halohydrins
- Practice Time - Halogenation
- Hydroboration/Oxidation of Alkenes: Hydration
- Practice Time - Hydroboration-Oxidation
- Oxymercuration-Reduction: Hydration
- Practice Time - Oxymercuration/Reduction
- Oxidation and Reduction in Organic Chemistry
- Calculating Oxidation States of Carbon
- Identifying oxidation and reduction reactions
- Practice Time - Oxidation and Reduction in Organic
- Oxidation
- Reduction
- Capsaicin
-
Alkynes
- Structure of Ethyne (Acetylene)
- Naming Alkynes
- Practice Time! - Naming Alkynes
- Physical Properties of Alkynes
- Preparation of Alkynes
- Practice Time! - Preparation of Alkynes
- H-X Addition to Alkynes
- X2 Addition
- Hydration
- Reduction of Alkynes
- Practice Time! - Addition Reactions of Alkynes
- Oxidative Cleavage of Alkynes
- Alkyne Acidity and Acetylide Anions
- Reactions of Acetylide Anions
- Retrosynthesis Revisted
- Practice Time! - Multistep Synthesis Using Acetylides
-
Alkyl Halides and Alcohol
- Naming Alkyl Halides
- Naming Alcohols
- Classes of Alcohols and Alkyl Halides
- Practice Time! - Naming Alkyl Halides
- Practice Time! - Naming Alcohols
- Physical Properties of Alcohols and Alkyl Halides
- Structure and Reactivity of Alcohols
- Structure and Reactivity of Alkyl Halides
- Preparation of Alkyl Halides and Tosylates from Alcohols
- Practice Time! - Alcohols to Alkyl Halides
- Preparation of Alkyl Halides from Alkenes; Allylic Bromination
- Strategy for Predicting Products of Allylic Brominations
- Practice Time! - Allylic Bromination
-
Substitutions (SN1/SN2) and Eliminations (E1/E2)
- Introduction
- Solvents
- SN1 Reaction: The Carbocation Pathway
- SN2 Reactions: The Concerted Backside Attack
- SN1 vs. SN2: Choosing the Right Path
- Application: Cardura (Doxazosin)
- E1 Reactions: Elimination via Carbocations
- E2 Reactions: The Concerted Elimination
- E1cB: The Conjugate Base Elimination Pathway
- Substitution versus Elimination
- Dienes, Allylic and Benzylic systems
-
General Chemistry Review
-
3.
Second Semester Topics
- Arenes and Aromaticity
-
Reactions of Arenes
- Electrophilic Aromatic Substitution
- EAS-Halogenation
- EAS-Nitration
- Practice Time - Synthesis of Aniline
- EAS-Alkylation
- Practice Time - Friedel Crafts Alkylation
- EAS-Acylation
- Practice Time - Synthesis of Alkyl Arenes
- EAS-Sulfonation
- Practice Time - EAS
- Donation and Withdrawal of Electrons
- Regiochemistry in EAS
- Practice Time - Directing Group Effects
- Synthesizing Disubstituted Benzenes: Effects of Substituents on Rate and Orientation
- Steric Considerations
- Strategies for Synthesizing Disubstituted Benzenes
- NAS - Addition/Elimination
- NAS - Elimination/Addition - Benzyne
- Alcohols and Phenols
-
Ethers and Epoxides
- Intro and Occurrence
- Crown Ethers and Cryptands
- Preparation of Ethers
- Reactions of Ethers
- Practice Time - Ethers
- Preparation of Epoxides
- Reactions of Epoxides - Acidic Ring Opening
- Practice Time - Acidic Ring Opening
- Reactions of Epoxides - Nucleophilic Ring Opening
- Practice Time - Nucleophilic Ring opening
- Application - Epoxidation in Reboxetine Synthesis
- Application - Nucleophilic Epoxide Ring Opening in Crixivan Synthesis
- Physical Properties of Ethers and Epoxides
- Naming Ethers and Epoxides
-
Aldehydes and Ketones
- Naming Aldehydes and Ketones
- Physical Properties of Ketones and Aldehydes
- Practice Time - Naming Aldehydes/Ketones
- Nucleophilic addition
- Addition of Water - Gem Diols
- Practice Time - Hydration of Ketones and Aldehydes
- Addition of Alcohols - Hemiacetals and Acetals
- Acetal Protecting Groups
- Hemiacetals in Carbohydrates
- Practice Time - Hemiacetals and Acetals
- Addition of Amines - Imines
- Addition of Amines - Enamines
- Practice Time - Imines and Enamines
- Application - Imatinab Enamine Synthesis
- Addition of CN - Cyanohydrins
- Practice Time - Cyanohydrins
- Application - Isentress Synthesis
- Addition of Ylides - Wittig Reaction
- Practice Time - Wittig Olefination
- Structure of Ketones and Aldehydes
- Carboxylic Acids and Derivatives
- Enols and Enolates
- Condensation Reactions
-
4.
NMR, IR, UV and MS
- Spectroscopy
-
HNMR
- Nuclear Spin
- Interpreting
- Chemical Shift
- Practice Time! - Chemical Shift
- Equivalency
- Indentifying Homotopic, Enantiotopic and Diastereotopic Protons
- Practice Time! - Equivalency
- Intensity of Signals
- Spin Spin Splitting
- Practice Time! - Spin-Spin Splitting
- Primer on ¹³C NMR Spectroscopy
- Alkanes
- Alkynes
- Alcohols
- Alkenes
- Coupling in Cis/Trans Alkenes
- Ketones
- HNMR Practice 1
- HNMR Practice 2
- HNMR Practice 3
- HNMR Practice 4
- Exchangeable Protons and Deuterium Exchange
- IR - Infrared Spectroscopy
- UV - Ultraviolet Spectroscopy
- Mass Spectrometry
-
5.
General Chemistry
- General Chemistry Lab
- Strategy for Balancing Chemical Reactions
- Calculator Tips for Chemistry
- Significant Figures
- Practice Time! Significant Figures
- Spreadsheets - Getting Started
- Spreadsheets - Charts and Trend lines
- Standard Deviation
- Standard Deviation Calculations
- Factor Labels
- Practice Time! - Factor Labels
- Limiting Reagent Problem
- Percent Composition
- Molar Mass Calculation
- Average Atomic Mass
- Empirical Formula
- Practice time! Empirical and Molecular Formulae
- Initial Rate Analysis
- Practice Time! Initial Rate Analysis
- Solving Equilibrium Problems with ICE
- Practice Time! Equilibrium ICE Tables
- Le Chatelier's
- Practice Time! Le Chatelier's Principle
- 6. Organic Chemistry Lab
- 7. Tools and Reference
-
8.
Tutorials
- Reaction Mechanisms (introduction)
- Factor Labels
- Acetylides and Synthesis
- Drawing Cyclohexane Chair Structures
- Drawing Lewis Structures
- Aromaticity Tutorial
- Common Named Aromatics (Crossword Puzzle)
- Functional Groups (Flashcards)
- Characteristic Reactions of Functional Groups
- Alkyl and Alkenyl Groups
- Valence Bond Theory
- Alkane Nomenclature
-
9.
The Alchemy of Drug Development
- Ivermectin: From Merck Innovation to Global Health Impact
- The Fen-Phen Fix: A Weight Loss Dream Turned Heartbreak
- The Asymmetry of Harm: Thalidomide and the Power of Molecular Shape
- Semaglutide (Ozempic): From Lizard Spit to a Once-Weekly Wonder
- From Cocaine to Novocain: The Development of Safer Local Anesthesia
- The Crixivan Saga: A Targeted Strike Against HIV
- The story of Merck’s COX-2 inhibitor, Vioxx (rofecoxib)
- The Accidental Aphrodisiac: The Story of Viagra
- THC: A Double-Edged Sword with Potential Neuroprotective Properties?
- Ritonavir Near Disaster and Polymorphism
- 10. Allied Health Chem
Clear History
Quick Menu
Flash Chromatography
Flash chromatography is a quick and efficient method for separating and purifying compounds in a mixture. Here's a step-by-step guide on how to run flash chromatography:
Materials Needed
- Flash chromatography column
- Solvent system (e.g., hexane, ethyl acetate)
- Sample mixture to be separated
- UV lamp or detector (if applicable)
- Collection tubes or flasks
Preparation
1. **Column Selection:** Choose a column with the appropriate size and stationary phase (e.g., silica gel) for your sample.
2. **Packing the Column:** Slurry pack the column with the stationary phase (e.g., silica gel) in a suitable solvent. Ensure that the column is packed evenly without air bubbles.
3. **Equilibration:** Pass a few column volumes of the eluent (solvent or solvent mixture) through the column to equilibrate the stationary phase.
Choosing a Stationary Phase
In flash column chromatography the most commonly used stationary phases are silica gel (SiO2) and alumina (Al2O3). Other stationary phases are available for specific uses (Table 1).
Figure 2 shows the general structure of normal phase, reversed phase and specialized silica. Normal phase silica has terminal silanol groups which give it its polar adsorption properties. In reversed phase silica, the silanol groups are replaced by apolar groups such as C-18 carbon chains, which makes it well suited for purification of highly polar compounds. The carbon chains can also be capped at their ends to produce specialized silica.
| Sample Characteristics | Stationary Phase | Conditions |
|---|---|---|
| Low to medium polarity |
Normal phase silica |
Normal Phase |
| High polarity |
Reversed phase silica |
Reverse Phase |
| Basic |
Basic alumina |
Normal Phase |
| Acidic |
Acidic alumina |
Normal Phase |
| Acid sensitive |
Neutral alumina |
Normal Phase |
| Charged |
Reversed Phase Silica |
Reversed Phase |
While adding Et3N or AcOH to the eluant can prevent streaking of basic and acidic compounds on normal phase silica, it also implies having to remove it from the purified product usually by extraction or high vacuum evaporation. Using a specialized silica with an acidic or basic moiety bound to it eliminates this problem. Alumina can also be used as a stationary phase.
Packing the Column
1. Preparation: Place a small piece of glass wool or a frit at the bottom of the column to prevent the stationary phase from escaping.
2. Adding Sand: Add a small layer of sand on top of the glass wool to create a flat surface for the stationary phase.
3. Slurry Packing: Prepare a slurry by mixing the stationary phase (e.g., silica gel) with an appropriate solvent. Pour the slurry into the column, ensuring that it settles evenly. Tap the column gently to remove air bubbles and achieve a uniform packing. Determine the silica gel to compound ratio. Easy separations require ratios between 30-50:1 (by weight), while harder separations call for ratios of up to 120:1.
4. Top Layer of Sand: Once the column is packed with the stationary phase, add another layer of sand on top. This layer helps to distribute the solvent and sample evenly and prevents disturbance of the silica gel during sample loading.
By following these steps, you can ensure that your flash chromatography column is properly packed for efficient separation.
Prepare and load your sample
There are three different ways to load a product onto a column: neat, in solution or dry.
- Neat is rarely used because it can overload the column and lead to cracking.
- Dry loading is seldom used because of the risks associated with it (acidity and volatility of silica gel).
- Loading of a solution of the product is by far the easiest way and will be described here.
Ideally the crude product should be dissolved into the least polar solvent possible such as hexane or pentane. Unfortunately, a lot of products will be insoluble in those solvents. In this case, the easiest is to dissolve the crude mixture into dichloromethane. Dichloromethane will dissolve most organic products while still being relatively non polar. The product should also be dissolved in the minimum amount of solvent in order to start with the thinnest band possible. If too much solvent is added it is best to concentrate it back down and start again.
Once the crude mixture is in solution, open the stopcock and using a long glass pipette carefully add the solution to your column by running it along the wall of the column as far down as you can. Once all the solution has been added, let it absorb onto the silica gel. Then rinse your crude material flask with a small volume of eluant and carefully add it to your column, rinsing the walls of the column. Let it absorb and repeat two more times. The idea is to get all the crude material absorbed onto the silica gel using the smallest amount of solvent possible so as to not dilute it too much.
After the three rinses, fill your column with eluant. At first using a pipette until you have a few centimeters of solvent then straight from the Erlenmeyer. Always run the solvent down the walls of the column to avoid disturbing your silica gel.
Running the column
With your column full of solvent you can start applying pressure. It is usually safe to elute the first column volume into an Erlenmeyer flask as your product should not come out until the third column volume (Rf=0.3 => CV=3.3). Once you've eluted a column volume, switch to your test tubes, filling each up to about 80-90%. Always make sure there is solvent above the silica. The column should never run dry. Once all your initial eluant is used up gradually increase the polarity until you get to twice the polarity of your TLC solvent. Increasing the polarity too rapidly will result in cracking of the silica and should therefore be avoided.
While you are running the column, you should be checking your fractions by TLC. This may be a little overwhelming at first, but eventually it will become easier. If it is too hard, you can stop the pressure while spotting, but constant changing of the pressure can also lead to cracks in the silica and should be avoided. When spotting TLCs, spotting every other tube usually gives you a good enough idea of the separation all while saving time and TLC plates. Once all the products of interest have eluted out of the column, you can safely flush and dry your column.
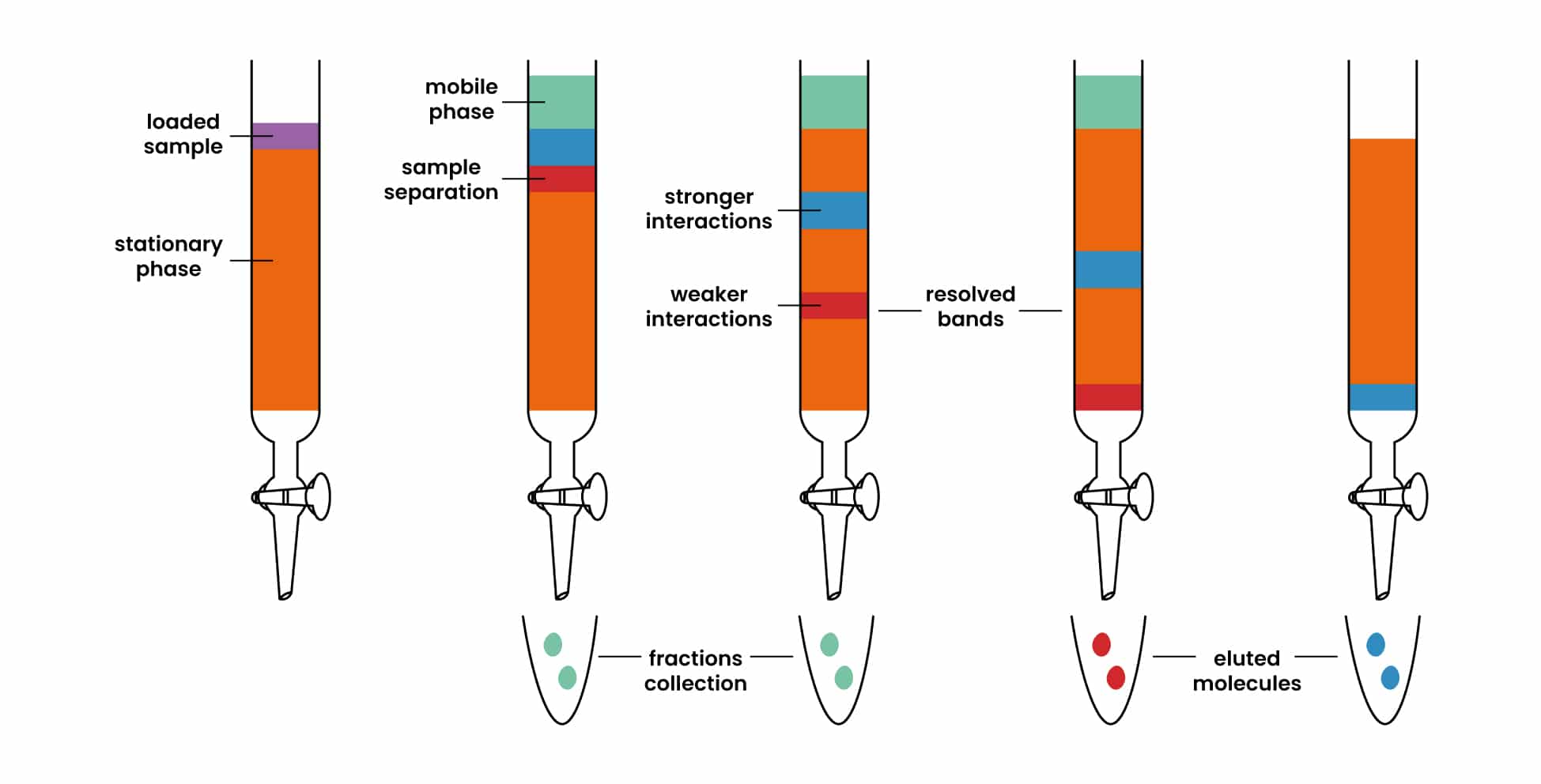
Fraction Collection
1. **Collect Fractions:** Collect the eluate in separate tubes or flasks. Label each fraction clearly.
2. **Analysis:** Analyze the fractions using techniques such as thin-layer chromatography (TLC) to identify the compounds present.
Cleaning and Storage
1. **Cleaning the Column:** After the separation is complete, wash the column with a suitable solvent to remove any residual compounds.
2. **Storage:** If the column is reusable, store it in an appropriate solvent to prevent drying out.
Safety Considerations
- Always wear appropriate personal protective equipment (PPE) such as gloves and safety glasses.
- Work in a well-ventilated area, especially when using volatile solvents.
- Dispose of solvents and waste materials according to your institution's safety guidelines.
By following these steps, you can effectively separate and purify compounds using flash chromatography. Adjustments may be necessary based on the specific requirements of your sample and the complexity of the mixture.